Recent Posts
THE STORY OF MY WILSON DISEASE: AGE 8 TO 53
By Barbara Noci I was born on the 6th of April 1972 in Empoli, a little city near Florence in [...]
Travis’s Story of Wilson Disease
Rare and Rural By Rhonda Rowland, WDA President We all love a small town. Images come to mind of holiday [...]
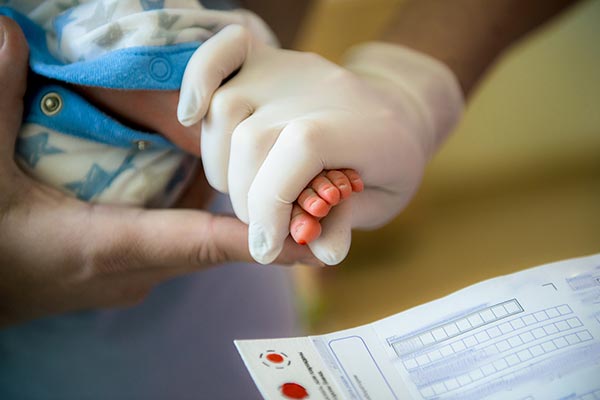
Newborn Screening for Wilson Disease
A 25-year dream coming close to reality By Alice Williams, WDA Communications Director Since the 1960s, the United States has [...]
Can Copper be Absorbed Through the Skin?
By Edward Tabor, MD, WDA Board Member The main source of copper for humans is food, and Wilson disease patients [...]
They Said “Yes” to Gene Therapy
By Rhonda Rowland, WDA PresidentWe continue the story of two trailblazers who are among the first Wilson disease (WD) patients [...]
A Makeover for an Old Drug
By Rhonda Rowland Wilson disease (WD) was always fatal until Dr. John Walshe, an English physician, discovered penicillamine in [...]
Ultragenyx Wilson Disease Update August 2023
On behalf of the Ultragenyx Wilson disease study team, we would like to share an update on our CYPRUS2+ study.
On behalf of the Ultragenyx Wilson disease study team, we would like to share an update on our CYPRUS2+ study.
Ultragenyx is pleased to announce that dosing has begun for the second group of patients in the CYPRUS2+ study, a one-time intravenous (IV) infusion of UX701, an investigational gene therapy for the treatment of Wilson disease.
The CYPRUS2+ study is designed with three stages. During the first stage, the safety and efficacy of up to three dose levels of UX701 will be evaluated over the course of 52 weeks and a dose will be selected for further evaluation in Stage 2. In this first stage, 15 patients will be enrolled into three dosing groups. In the first group of patients, UX701 has been well tolerated with no serious adverse events. Additional safety data is being evaluated and is expected in the first half of 2024. The first patient in the second group has received a dose of UX701 and the remaining four patients have been identified.
Ultragenyx is sponsoring this global study to determine if UX701 gene therapy is a safe and effective treatment for adults with Wilson disease. This adeno-associated virus (AAV) gene therapy has the potential to restore ATP7B function, which may improve copper metabolism and distribution. UX701 is an investigational product for which safety and effectiveness as a treatment for Wilson disease has yet to be proven. Stage 1 of the Phase 1/2/3 trial is on track to be completed this year.
The CYPRUS2+ study continues to enroll adults living with Wilson disease, currently well-managed on standard of care. We will be enrolling across multiple sites in the U.S., Canada, and Europe. You are invited to email PatientAdvocacy@Ultragenyx.com for more information. Please find a full summary of the study at: https://clinicaltrials.gov/ct2/show/NCT04884815?term=ux701&draw=2&rank=1
Ultragenyx is grateful to those individuals who have participated, or are currently participating, in our Wilson disease research. These experiences and insights help advance our understanding of WD and have the potential to move research forward for future generations. We will continue to share updates as new information becomes available.