Recent Posts
THE STORY OF MY WILSON DISEASE: AGE 8 TO 53
By Barbara Noci I was born on the 6th of April 1972 in Empoli, a little city near Florence in [...]
Travis’s Story of Wilson Disease
Rare and Rural By Rhonda Rowland, WDA President We all love a small town. Images come to mind of holiday [...]
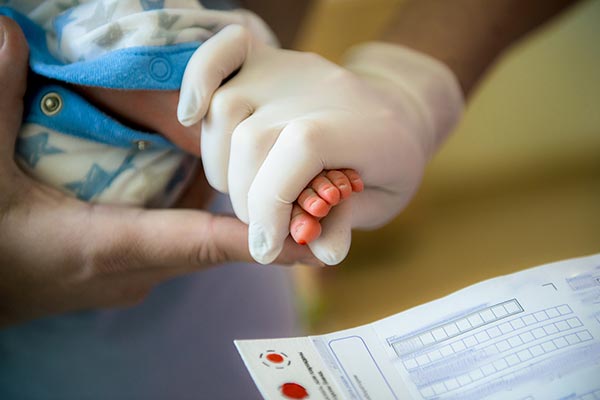
Newborn Screening for Wilson Disease
A 25-year dream coming close to reality By Alice Williams, WDA Communications Director Since the 1960s, the United States has [...]
Can Copper be Absorbed Through the Skin?
By Edward Tabor, MD, WDA Board Member The main source of copper for humans is food, and Wilson disease patients [...]
They Said “Yes” to Gene Therapy
By Rhonda Rowland, WDA PresidentWe continue the story of two trailblazers who are among the first Wilson disease (WD) patients [...]
A Makeover for an Old Drug
By Rhonda Rowland Wilson disease (WD) was always fatal until Dr. John Walshe, an English physician, discovered penicillamine in [...]
Alexion’s Wilson Disease Drug Program Terminated
April 28, 2023
The Wilson Disease Association (WDA) is disappointed to share that Alexion, AstraZeneca Rare Disease decided to terminate its ALXN1840 program. Alexion pursued rigorous scientific research in its effort to bring forward a novel therapy for Wilson disease (WD) patients. Unfortunately, additional studies done to confirm the ALXN1840 mechanism of action did not achieve their endpoints, and they do not see a path forward to achieve approval from the Food and Drug Administration (FDA) or European Medicines Agency (EMA).
“We would like to acknowledge the tremendous contribution of the families who participated in these studies, as well as the broader WD community for their collaboration. Without such dedication, advancing meaningful research would not be possible,” said Wendy Erler, VP Patient Experience, Alexion. “We acknowledge this is terribly disappointing news for the entire WD community and not the outcome we were hoping for.”
Alexion has notified all clinical trial investigators about the decision and provided guidance on next steps. Following discontinuation of ALXN1840, the treating physician will discuss with each patient the treatment plan going forward, including transitioning to the appropriate standard of care medication. The medication transition will be individualized to each patient based on the clinical judgment of the treating physician.
“We know that many aside from ourselves will be disappointed by this development and the rapidity of the announcement,” said Dr. Michael Schilsky, Study Investigator, Yale School and Medicine and Chair, WDA Medical Advisory Committee. “Please be assured that we will do our best to make sure each patient is cared for properly in this transition.”
While the end result may be disappointing, there is reason for hope. The medical data gathered from these studies may be helpful in general for understanding more about WD and copper metabolism. It’s also advanced the understanding of the challenges in conducting clinical trials for WD.
“It is our opinion that many patients benefitted from the study treatment,” said Dr. Schilsky, “and this perhaps is our biggest disappointment in not seeing this succeed in safely jumping the regulatory hurdles.”
The WDA would like to thank the clinical trial investigators and the patients who participated in the ALXN1840 trials for the extensive time and effort they contributed to increasing the understanding of this rare, genetic liver disease. Treatment advances and better care in WD cannot be made without dedicated physicians and patient volunteers and the commitment that’s needed for new therapy trials to be successful.