Recent Posts
THE STORY OF MY WILSON DISEASE: AGE 8 TO 53
By Barbara Noci I was born on the 6th of April 1972 in Empoli, a little city near Florence in [...]
Travis’s Story of Wilson Disease
Rare and Rural By Rhonda Rowland, WDA President We all love a small town. Images come to mind of holiday [...]
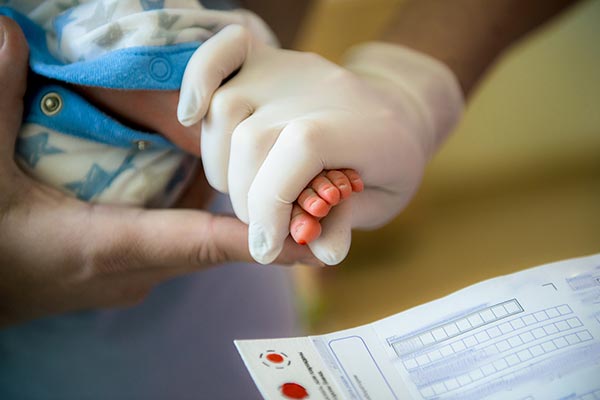
Newborn Screening for Wilson Disease
A 25-year dream coming close to reality By Alice Williams, WDA Communications Director Since the 1960s, the United States has [...]
Can Copper be Absorbed Through the Skin?
By Edward Tabor, MD, WDA Board Member The main source of copper for humans is food, and Wilson disease patients [...]
They Said “Yes” to Gene Therapy
By Rhonda Rowland, WDA PresidentWe continue the story of two trailblazers who are among the first Wilson disease (WD) patients [...]
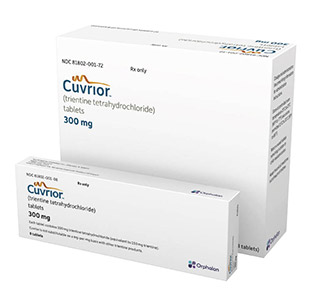
A Makeover for an Old Drug
By Rhonda Rowland Wilson disease (WD) was always fatal until Dr. John Walshe, an English physician, discovered penicillamine in [...]
Orphalan Partners with MAP International and the Wilson Disease Association to Provide Life-Changing Medicine to Underserved Communities
Orphalan SA, a global pharmaceutical company focused on rare diseases, is partnering with MAP International and the Wilson Disease Association (WDA) to provide Cuvrior® (trientine tetrahydrochloride) to patients with Wilson Disease in developing countries. Through this partnership, Orphalan will donate a supply of Cuvrior® to MAP International, a global humanitarian health organization with over 70 years of experience distributing critical medicines to communities in need.