Recent Posts
THE STORY OF MY WILSON DISEASE: AGE 8 TO 53
By Barbara Noci I was born on the 6th of April 1972 in Empoli, a little city near Florence in [...]
Travis’s Story of Wilson Disease
Rare and Rural By Rhonda Rowland, WDA President We all love a small town. Images come to mind of holiday [...]
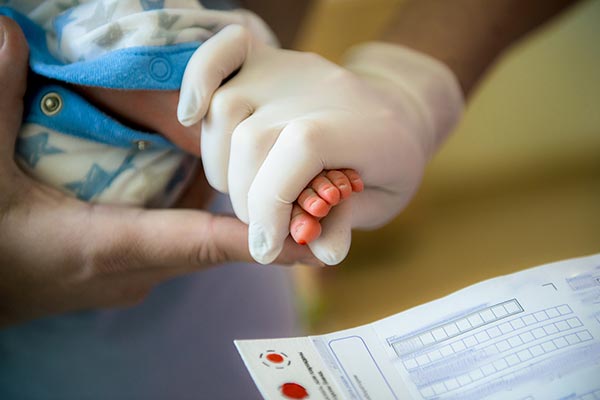
Newborn Screening for Wilson Disease
A 25-year dream coming close to reality By Alice Williams, WDA Communications Director Since the 1960s, the United States has [...]
Can Copper be Absorbed Through the Skin?
By Edward Tabor, MD, WDA Board Member The main source of copper for humans is food, and Wilson disease patients [...]
They Said “Yes” to Gene Therapy
By Rhonda Rowland, WDA PresidentWe continue the story of two trailblazers who are among the first Wilson disease (WD) patients [...]
Meet the Inaugural Gene Therapy Candidates
By Rhonda Rowland, WDA President The two gene therapy clinical trials for Wilson disease (WD) launched by Ultragenyx and [...]
Share Your Treatment Experience
Researchers from the Department of Medical Social Sciences at Northwestern University’s Feinberg School of Medicine in Chicago, IL, are seeking US-based patients with Wilson Disease (WD) to participate in a study on treatment experiences and preferences. This study aims to develop a questionnaire for use in WD treatment clinical trials conducted by Orphalan SA.
The questionnaire will be important in Orphalan’s efforts to expand treatment options for patients with WD. The title of the study is, Wilson Disease Treatment Experience Measure Development.
You may be eligible to participate if you take penicillamine (also called Cuprimine® or Depen®) or trientine (also called Syprine®or Cuvrior), or zinc (also called Galzin) and have been on the same dose for the past 3 or more months. If you are interested in participating, a member of the study team will confirm your eligibility in a brief telephone call. Eligible individuals would complete either 1 or 2 one-on-one telephone or Zoom-based interviews, each approximately 90 minutes in duration, during which you may be asked about your experiences with your WD treatments. You may also be asked to complete a questionnaire about WD treatment administration preferences and answer some questions afterward about the clarity and appropriateness of the questionnaire items. Interviews will be audio-recorded for later transcription and analysis. You will be in the study for the duration of time it takes to complete your interview(s). You will be compensated $125 USD in the form of an electronic Visa gift card after your interview(s).
To learn more about the study or to schedule an interview, please complete this contact form and a member of our team will reach out to you within one week.